Stronger connections in key brain networks appear to accelerate the spread of tau tangles, potentially worsening memory and language symptoms.
Many people with Alzheimer’s disease first lose neurons in their medial temporal lobe — a region of the brain involved in memory, navigation, emotional regulation, and language comprehension. So, one might expect a person with the disease to show reduced signaling activity in and around that part of the brain as Alzheimer’s progresses.
But according to a recent study in Brain, the opposite appears to be true: In fact, people with Alzheimer’s showed a unique and consistent pattern of increased connectivity in the medial temporal lobe.
Researchers at the University of Caen Normandy looked closely at two neural networks that connect the medial temporal lobe to the rest of the brain: the anterior-temporal (AT) system (which supports language comprehension) and the posterior-medial (PM) system (which is more involved in memory storage and retrieval).
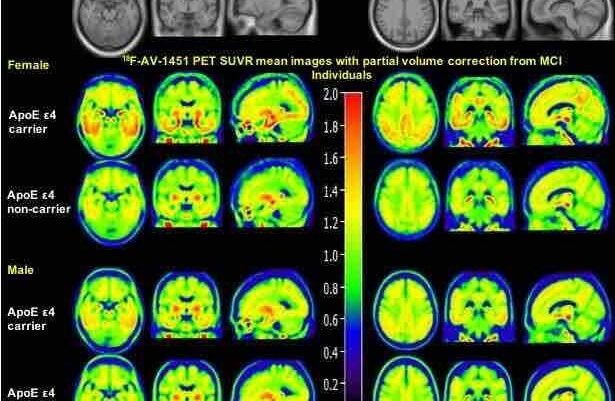
Neuroscientist and study co-author Robin de Flores and his team wanted to track how Alzheimer’s disease affected connectivity in each network, so they took fMRI scans of people with and without the disease. They enrolled 261 participants in total, including 209 people without any signs of cognitive impairment and 52 people with either mild cognitive impairment or dementia caused by Alzheimer’s. The cognitively unimpaired people ranged in age from 19 to 85, which helped de Flores’s team determine how normal aging affected each network as well.
They found that, across the board, older people tended to show lower connectivity in their PM network, but people living with Alzheimer’s stood out: They had uniquely high signaling activity in their AT network. And that higher AT connectivity was associated with poorer performance on cognitive assessments like the mini-mental state examination and the Mattis dementia rating scale.
Furthermore, de Flores’s team tracked the participants with MCI for seven years longer than the rest of the participants and saw that those with higher AT activity progressed to dementia more quickly.
Considering the typical symptoms of Alzheimer’s, these results were a bit surprising to de Flores. “We were expecting, actually, more effects of Alzheimer’s on the PM network because it’s involved in episodic memory,” he told Being Patient.
But the story started to make more sense when he turned his attention to the pathogenic proteins that drive neurodegeneration in the disease. In a previous study, de Flores found that each network seems to associate with a different protein as Alzheimer’s progresses. While amyloid plaques often cluster around the PM network, tau tangles — which are more closely linked to neurodegeneration — preferentially form inside of AT neurons.
As misfolded tau proteins accumulate inside one neuron, stronger network connectivity provides these proteins with more opportunity to spread to another.
“It’s like the neuron starts sneezing, and all of a sudden infecting its neighbors more easily,” Nicolai Franzmeier, a neuroscientist at the Ludwig Maximillian University of Munich, told Being Patient.
Franzmeier was not involved in de Flores’s study, but he recently published similar results in Science Translational Medicine. Franzmeier’s team examined fMRI and PET scans from hundreds of people in the Alzheimer’s Disease Neuroimaging Initiative and the A4 Study. But instead of focusing on specific brain networks, they pinpointed where tau first formed in each individual’s brain, then tracked how quickly it spread. The protein consistently spread most quickly to the regions of the brain with strongest connections to the original site.
Franzmeier and his team also highlighted a likely accelerator: beta-amyloid. They showed that people with higher levels of beta-amyloid had stronger connectivity between their tau epicenter and the regions of the brain to which tau later spread.
While de Flores did not collect data that would enable him to prove that beta-amyloid was the cause of the increased connectivity in the AT network, he thinks Franzmeier’s results provide compelling evidence for that argument.
“Tau really tells you what the symptoms will be,” he told Being Patient. “But amyloid also plays a very important role here, really acts as a booster for tau spreading.”
Both de Flores and Franzmeier believe their work points to a potential treatment strategy: antiepileptics — which reduce excessive neuronal activity — could offer a promising approach to treating the hyperconnectivity that beta-amyloid appears to induce.
According to Franzmeier, the key would be administering these drugs early, before tau has had a chance to spread and cause symptoms. If he can find evidence that this strategy actually benefits people with Alzheimer’s, he envisions a future where people keep their tau in check with a combination of amyloid-targeted therapies and low doses of seizure medications. “Because it’s such a complex disease, one drug will not solve the problem,” he said.
Andrew Saintsing (@AndrewSaintsing) earned a PhD in biology, and now he writes about science for outlets like Drug Discovery News.